|
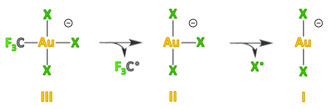
The mononuclear gold(II) halide complexes [AuCl3]− and [AuBr3]− are formed in the gas phase by collision-induced homolytic splitting of the only Au−C bond in the monoalkylgold(III) precursors [CF3AuX3]−. The geometries of the whole series of [AuX3]− complexes (X=F, Cl, Br, I) have been calculated by DFT methods. It has also been found that the neutral AuX2 molecules behave as unsaturated species, showing significant affinity for an additional X− ligand. Moreover, in the open-shell [AuX3]− anions, homolytic splitting of one of the Au−X bonds and formation of the lower-valent [AuX2]− anions is favored over non-reducing halide dissociation. They should therefore be prone to disproportionation.
|