|
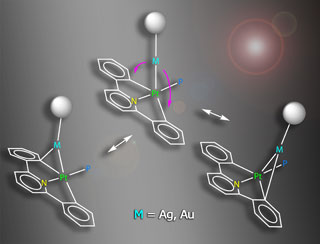
Heteropolynuclear complexes [(CNC)(PPh3)PtM(PPh3)](ClO4) (M = Au (1), Ag (2)) and [{Pt(CNC)(PPh3)}3M](ClO4) (M = Ag (3), Au (4); CNC = 2,6-diphenylpyridinate) have been prepared and studied by structural, spectroscopic techniques and DFT calculations. The X-ray crystal structures of 1, 3 and 4 confirm the existence of Pt-M bonds and M-Cipso interactions involving one of the phenyl fragments of CNC. Their NMR spectra confirm the persistence of the Pt-M interactions in solution and also show an intramolecular "metronome-like" dynamic processes consisting of back-and-forth bouncing of the acidic M fragments along the C-Pt-C axis. DFT calculations on these complexes identify two main orbital interactions between the [PtCNC] and [M]+ fragments, namely a donation of the former to a vacant orbital of the latter, and a much weaker backdonation from the acidic M to the Pt fragment. Overall, the strength of the [Pt]...M interactions is higher for the gold compounds than for their silver counterparts. The interaction between the acidic center (silver or gold) and the carbon atom of one of the phenylene rings in these heteropolymetallic complexes can be envisaged as the first step in a process of interchange of aryl ligands. However, the ligand exchange cannot progress further due to the polydentate nature of the CNC ligand, and therefore these structures can be considered as frozen "snapshots" of a transmetalation reaction which have been arrested at different steps in the process.
|