|
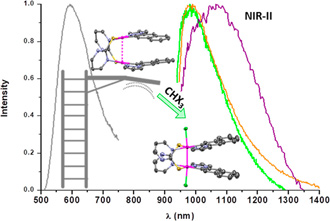
Half-lantern Pt(II) dinuclear complexes [{Pt(C∧Npz)(μ-S∧NR)}2] (HC∧Npz = 1-naphthalen-2-yl-1H-pyrazole; R = H, HS∧N: 2-mercaptopyrimidine 1; R = CF3, HS∧NF: 4-(trifluoromethyl)-2-mercaptopyrimidine 2) were selectively obtained as single isomers with the C∧N groups in an anti-arrangement and rather short metallophilic interactions (dPt–Pt = 2.8684(2) Å for 2). They reacted with haloforms in the air and sunlight to obtain the corresponding oxidized diplatinum(III) derivatives [{Pt(C∧Npz)(μ-S∧NR)X}2] (X = Cl (1-Cl), Br (1-Br), I (1-I, 2-I)). The single-crystal X-ray structures exhibit Pt–Pt distances typical for the existence of a metal–metal bond, which evidence fairly well the influence of the axial ligand (X). The reactions of 1 and 2 with CHI3 in the dark afforded mixtures of [IPt(C∧Npz)(μ-S∧N)2Pt(C∧Npz)CHI2] and 1-I or 2-I, with the former being the major species under an Ar atmosphere, while the reactions of 1 with CHBr3 and CHCl3 need light to occur. These Pt2(III,III) complexes display low-energy absorptions and emissions that strongly depend on the axial ligand. In the solid state, they show a broad NIR emission ranging from 985 to 1070 nm at RT that suffers a hypsochromic shift when cooling down to 77 K. The photoemissive behavior of the dinuclear Pt(II) and Pt(III) systems is disclosed with the aid of density functional theory calculations.
|