|
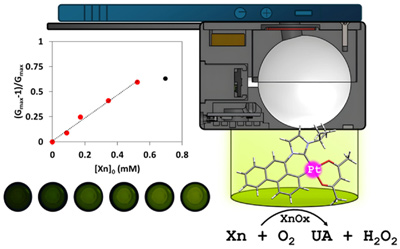
A cyclometalated N-heterocyclic carbene platinum(II) complex comprising a phenanthrenyl moiety has been prepared and used to synthesize neutral and cationic luminescent complexes containing different ancillary ligands: [Pt(Phen^C*)(acac)] (5), [Pt(Phen^C*)(CNXyl)2]PF6 (6) and [Pt(Phen^C*)(P^P)]PF6 (P^P: 1,1-bis(diphenylphosphino)methane, dppm 7; 1,2-bis(diphenylphosphino)ethane, dppe, 8, 1,2-bis(diphenylphosphino)benzene, dppbz, 9). Structural characterization by NMR and single-crystal X-ray studies endorsed the proposed molecular structure for these complexes with the formation of a six-membered platinacycle. Photophysical and computational studies for 5-9 disclosed the ligand-centered nature of the triplet state, mainly governed by the extended aromatic system of the Phen^C* ligand. Immobilized in ethyl cellulose (EC) films, compound 5 shows elevated quantum yield and long decay times in argon atmosphere and high sensitivity to molecular oxygen. Blends of 5 and EC (C5) were tested as a luminescent oxygen probe for the determination of xanthine (Xn), showing good photo stability and reversibility. The RSD is 0.9 % (n = 3) for 3x10-4 M and the detection limit achieves values of -2x10-5 M. This platinum-based sensor delivers better results than the ones obtained with [Ru(bpy)3]Cl2.6H2O, a typical luminophore used in commercial oxygen sensors. To improve the feasibility of the Xn determination, the sensor was implemented in a smartphone adapted device to be used as a in-situ Xn control.
|