|
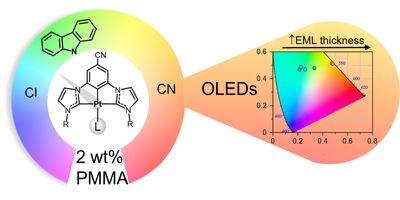
A set of neutral platinum(II) complexes bearing a bis-N-heterocyclic carbene (NHC) pincer ligand with stoichiometry [Pt(CR*^CCN^CR*)L] (R = Me (a), Et (b); L = Cl (2), Cbz (3), and CN (4)) have been prepared. By employing the corresponding bisimidazolium salt (1a or 1b), silver oxide and [PtCl2(cod)] we obtained the Cl derivatives. Then, the subsequent ancillary ligand exchange rendered complexes 3 and 4. Single-crystal X-ray analysis revealed no Pt-Pt contacts, but some π-π intermolecular interactions within the supramolecular structure of the Cl and CN derivatives. It also showed different crystal packings for the two polymorphs of the Cl complex (2b). In diluted solution, photophysical and computational studies disclosed the nature of the low-lying electronic transitions to be mainly 3ILCT [π(C*^C^C*) → π*(C*^C^C*)]/3MLCT [5d(Pt) → π*(C*^C^C*)] for Cl and CN derivatives and L'LCT [π(Cbz) → π*(C*^C^C*)]/L'MCT [π(Cbz) → d(Pt)] for the Cbz counterpart. The blue (2b), green (3b) and yellowish-orange (4b) emissions of 2 wt% PMMA films at λex = 370 nm exhibited very high quantum yields (QYs) reaching up to ≅99%. However, at λex > 400 nm, in the solid state or in 20 wt% PMMA films, additional structureless low-energy emissions arose from extended structures with a higher degree of aggregation. Complexes 2b-4b were used as emitters in organic light-emitting diodes (OLEDs), either with a doped or a non-doped emissive layer. Interestingly, and due to their aggregation in extended structures, their emission could be tuned from bluish green to orange just by changing the thickness of the active layers. Devices with 4b reached a maximum efficiency of 8.08% and a high luminance of 34 071 cd m-2.
|