|
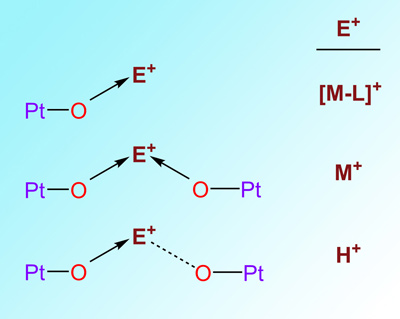
The Lewis base (LB) properties of the d8 Pt(II) are manifested in the formation of Pt→E species, where E are electrophiles such as acidic hydrogens or metal cations. Thus, the reactions of [Pt(CNC)(dmso)] (CNC = 2,6-di(phen-2-ide)-pyridine) toward PPh2(C6H4-o-COOH) or PPh2(C6H4-o-OH) produce complexes [Pt(CNC-H){PPh2(C6H4-o-COO)}] (1) and [Pt(CNC-H){PPh2(C6H4-o-O)}] (2). Their X-ray structures confirm the proton transfer from the OH at the phosphane to one of the phenylene rings of the CNC ligand. Then, the deprotonated oxygen occupies the vacant site. It is proposed that the transfer starts with a hydrogen bond (Pt→H) which evolves to the final products favored by the planar nature of the CNC ligand. 1 and 2 react with Ag+ and [M(PPh3)]+ (M = Ag, Au) giving rise to complexes without Pt-M bonds, but O-M or O-Ag-O instead. Their structures reveal that the oxygen atoms present in 1 and 2 advantageously compete with the Pt as the donor entity for the incoming acidic metal. Moreover, dinuclear complexes with O-H...O systems structurally analogous to the O-Ag-O have been prepared and characterized. They reinforce the idea of resemblance of the proton and cations such as Ag+, Au+, [AgL]+, and [AuL]+ as Lewis acidic moieties, which is consistent with the concept of isolobality.
|